Water naturally contains numerous salts. Some of them, which are very important, are Calcium salts (also known as Hardness) which abundantly exist in groundwater resources. These salts exist in water in the form of Bicarbonates. Calcium bicarbonates ( Ca(HCO3)2 ) are highly water-soluble; however, they are sensitive to thermal changes and when the temperature rises or evaporation happens, they decompose into Carbonates. Due to their unstable nature, bicarbonates are also known as temporary hardness. Calcium carbonate ( CaCo3 ) is not sensitive to temperature, thus it is called permanent hardness, but it is very little water-soluble.
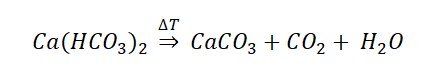
Scale is the result of water-soluble bicarbonates transformation into insoluble carbonates. Insoluble carbonates form hard lime scales on equipment walls.